What is DES?
Diethylstilbestrol: Introduction
Diethylstilbestrol is known under many different names, but the most common abbreviation is "DES", which is why we often use it on the website.
Diethylstilbestrol is the best known synthetic estrogen. It was synthesized in 1938 by E.C Dodds. Find out more about the synthesis of diethylstilbestrol at DES Timeline.
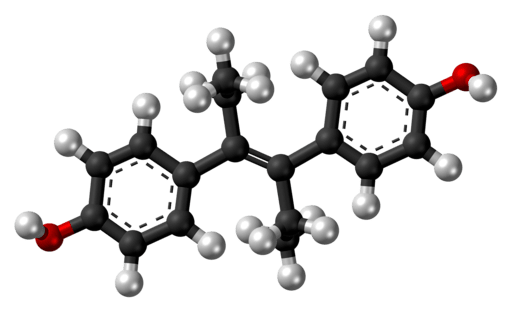
Formula: C18H2002
Other names: DES; Stilboestrol; Stilbestrol
Diethylstilbestrol appears as an odorless white powder made up of colorless crystals1.
Diethylstilbestrol has a melting point of 169 -172 ° C (336 to 342 °F). It is practically insoluble in water and chloroform, soluble in ethanol, methanol, benzene, light petroleum ether, chloroethane, acetone, and potassium hydroxide, and very soluble in oils and fats2.

If diethylstilbestrol is the most known synthetic hormone, it is not the first to have been synthesized and to have been given to pregnant women.
Paroxypropione was synthesized in 1902, but its antigonadotropic properties were only discovered in 1951.
It was marketed in France by Laroche-Navarron laboratories under the brand name Frénantol® from 1953, and, if it was mainly used to treat hyperthyroidism-related problems, it was also prescribed to prevent late spontaneous abortions associated with an hypersecretion of luteinizing hormone3. However, it did not have a very strong estrogenic activity and was not marketed for long, unlike diethylstilbestrol.
We do not know if it may have been consequences, but what we can say is that paroxypropione is a chemical precursor in the synthesis of diethylstilbestrol and dienestrol.

Laroche-Navarron laboratories
Exploitation of diethylstilbestrol
Since its inventor, Dr. Dodd’s, has not filed a patent, diethylstilbestrol has been produced by different laboratories and marketed in France and other countries under different various brand names (Stilbœstrol-Borne®, Distilbene® and Furostilbœstrol® in France) and in different forms: tablets, injections, creams, vaginal suppositories...
Other synthetic estrogens similar to diethylstilbestrol were marketed at the same time, notably dienestrol and hexestrol.
Examples of medication boxes



Tube of 20 dienœstrol tablets
By Bruneau & C Laboratories
Examples of drug advertising campaigns that were sent to doctors


Published by Fraysse & C Laboratories
Illustration by Albert DUBOUT
DiEthylStilbestrol (DES): a synthetic estrogen
The body makes three major forms of estrogens:
-
estrone or œstrone (E1)
-
estradiol or œstradiol (E2)
-
estriol or œstriol (E3)
They are present in both men and women, just in differing amounts.
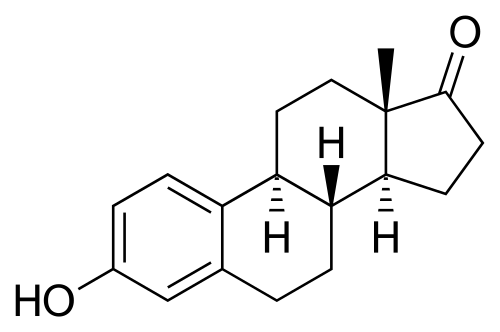
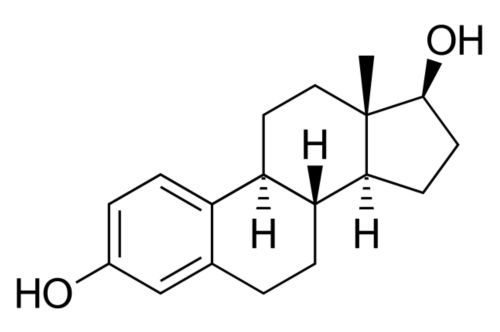
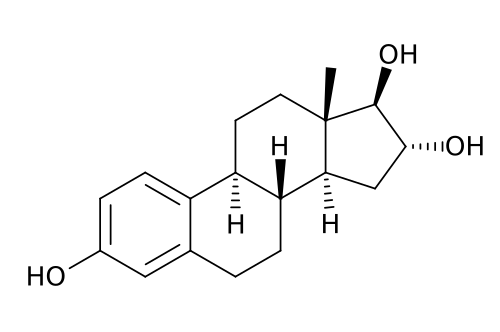
These natural estrogens contain, like all steroids, a sterol nucleus.
Although they can mimic their effects by binding to hormone receptors, synthetic hormones have nothing to do with the natural hormones produced by the human body. These substances are foreign can interfere with the hormone signaling in different ways and cause many adverse effects.
Diethylstilbestrol do not have a steroidal chemical structure: it is a non-steroidal estrogen.
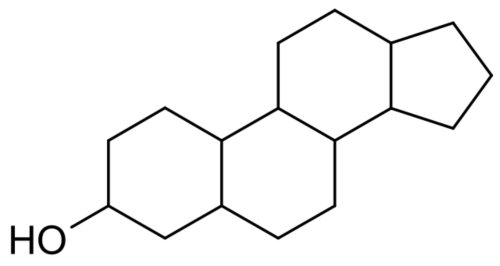
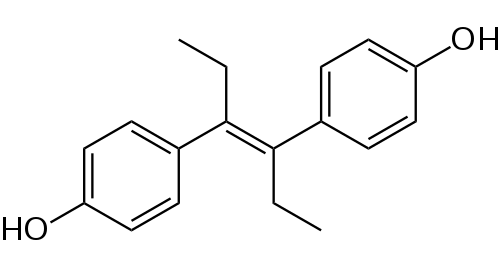
Non-steroidal estrogens are teratogenic, from the Greek teras, teratos, "monster" and genos, "origin", literally meaning, "monster making".
A so-called “teratogenic” substance is harmful because it can disrupt fetal development and cause congenital malformations in children exposed during the fetal period.
List of synthetic non-steroidal estrogens that have been used that are chemically similar to diethylstilbestrol4
Non Steroidal Estrogens
| Benzestrol | Fonatol | Restrol |
| Chlorotrianisene | Gynben | Stil-Rol |
| Comestrol | Gyneben | Stibale |
| Cyren A. | Hexestrol | Stilbestrol |
| Cyren B. | Hexoestrol | Stilbestronate |
| Delvinal | Hi-Bestrol | Stilbetin |
| DES | Menocrin | Stilbinol |
| DesPlex | Meprane | Stilboestroform |
| Dibestil | Mestilbol | Stilboestrol |
| Diestryl | Methallenestril | Stilboestrol DP |
| Dienestrol | Microest | Stilestrate |
| Dienoestrol | Mikarol | Stilpalmitate |
| Diethylstilbestrol Dipalmitate | Mikarol forti | Stilphostrol |
| Diethylstilbestrol Diphosphate | Milestrol | Stilronate |
| Diethylstilbestrol Dipropionate | Monomestrol | Stilrone |
| Diethylstilbenediol | Neo-Oestranol I | Stils |
| Digestil | Neo-Oestranol II | Synestrin |
| Domestrol | Nulabort | Synestrol |
| Estilben | Oestrogenine | Synthoestrin |
| Estrobene | Oestromenin | Tace |
| Estrobene DP | Palestrol D. | Vallestril |
| Estrosyn | Palestrol | Willestrol |
Non Steroidal Estrogen-Androgen Combinations
| Amperone | Metystil | Tylandril |
| Di-Erone | Teserene | Tylostrerone |
| Estran |
Non Steroidal Estrogen-Progesterone Combinations
- Progravidium
Vaginal cream – suppositories with Non Steroidal Estrogen
- AVC Cream with Dienestrol
- Dienestrol Cream
Diethylstilbestrol Indications
Betwen 1948 and1980
In human medicine
Diethylstilbestrol (DES) has been prescribed for a wide range of indications:
- To prevent miscarriages and premature births, until 1977 (in France);
- Gestational diabetes5;
- Inhibition of lactation (artificial suppression of the milk supply after delivery) - Distilbene® (diethylstilbestrol) or Cycladiene® (hexadiene), possibly combined with androgens, until 1980 (in France)6;
- Hypogalactia (low milk supply during breast-feeding);
- To treat estrogen deficiency;
- In case of amenorrhea (by the age of 16);
- To avoid painful periods and dysmenorrhea (menstrual cramps);
- To treat gynecological menorrhagia;
- Vaginitis (inflammation of the vagina);
- Breast hypoplasia (underdeveloped breasts);
- Breast ptosis (sagging breasts);
- Breast cancer;
- To treat pre-menopausal and menopausal symptoms, such as migraines, insomnia, hot flashes, venous insufficiency, eczema, osteoporosis, rheumatism or vulvar pruritus (itchy vulva);
- Pubertal disorders, such as acne7;
- Genital infantilism (underdevelopment of the genitals);
- Psychoses;
Since DES was also sold on the black market8, anecdotally, transgender people have consumed it without any medical control9;
It has also reportedly been prescribed as an anabolic agent among athletes10;
Abroad, it has been prescribed to pre-teem girls who were at risk of becoming "too tall" to inhibit growth11.

(Reference: Le diéthylstilbestrol : Mise au point des connaissances en 2002)
In veterinary medicine
In early 194712, synthetic hormones, such as diethylstilbestrol (DES), were administered to cattle, either as feed additives or as implants. The objective was clear: to fatten them up. Indeed, the anabolic properties of diethylstilbestrol allowed to increase their growth by promoting water retention in the muscles.
More meat, more profits.
Since the scandal only broke out in the early 1980s, many people consumed it without their knowledge.
The use of synthetic estrogens was a fraud because it can be assumed that the consumer who bought the meat would not have bought it if he had known it contained estrogens, especially as no scientific research had been carried out to assess the impact on consumer health.
Sadly, the adverse health effects of eating hormone-treated meat were later widely known.

For use with hexoestrol implantation tablets 15mg strenght.
Nowadays
Abroad, in countries such as China or Thailand, it seems to be easy to obtain diethylstilbestrol (DES). For example, online stores offer it to sportsmen and women as an anabolic agent13.
In the United States and other countries, DES is used to treat urinary incontinence in dogs and cats, and benign prostatic hyperplasia in dogs14.
In France and abroad, DES is still used to treat resistant prostate cancers in men. In France, it is now its only indication; in addition to being poorly tolerated, it does not bring any improvement in the treatment of prostate cancer15.
Mechanism of Action of Diethylstilbestrol
Diethylstilbestrol has estrogenic activity similar to estrone but more active than estrone: 0.3 mg diethylstilbestrol is equivalent to the effect of 1 mg estrone. This drug surpasses both estrone and hexestrol.
Diethylstilbestrol was prescribed precisely for this potent estrogenic activity.
Therefore, among symptoms of DES exposure are those of hyperestrogenism: early puberty, premenstrual syndrome (PMS), endometriosis, risk of developing benign or malignant estrogen-dependent tumors...
High estrogen levels are clearly linked to an increased risk of obesity and cancer. Diethylstilbestrol is fat-soluble and thus can accumulate in fatty tissues; therefore, DES has an obesogenic action16,17.
Diethylstilbestrol is one of a number of substances that cross the placental barrier. As a result, Distilbene® (a french medicine containing diethylstilbestrol) ingested by pregnant women not only poisoned them, but also directly poisoned the child they were carrying, and just as much poisoned the future children of that child because their germ cells were also exposed to the drug.
This is what D.E.S is it sadly call "the Matryoshka effect of DES".
Thus, it is indeed three generations that have been directly exposed to diethylstilbestrol. That’s why congenital urogenital anomalies are found in the three generations.
The gonads are a vulnerable target for endocrine disruptors such as diethylstilbestrol. This is why we find in people exposed in utero: disorders of sex differentiation (DSD, ambiguous genitalia, pseudohermaphroditism, abnormal secretion of testosterone, hypersecretion of androgens...).

Diethylstilbestrol: a Model of Endocrine Disruptor
Diethylstilbestrol (DES), like other stilbenes, is carcinogenic and genotoxic in both animals and humans.
It is an endocrine disruptor. Endocrine disruptors are believed to act by altering the mechanisms of hormone action, and thus the normal functioning of the human body.
Diethylstilbestrol creates iatrogenic (from the Ancient Greek ἰατρός, "physician", and γένεσις, "origin", literally meaning "caused by the physician") side effects in the descendants of women who took DES.
It is now known that fetal exposure to endocrine disruptors may affect neurodevelopment and increase risk of developing psychiatric disorders.
D.E.S is it demands that diethylstilbestrol be definitively banned, for any use whatsoever, human or animal, in France and worldwide, in order to ensure the preservation of human reproduction: the very foundation of life.
References
- Jean-Michel Baronnie - Thèse pour le doctorat vétérinaire - Le diéthylstilboestrol - Recherche dans les preparations injectables et dans les carcasses de veau, 1973.
- E. Daubresse, E.C Dodds, H. Scribe, CL Beclere, J. Peters; LE STILBOESTROL, Actualités Médicales n°4 Conférence de Bruxelles, 9 mars 1947.
- Laboratoires Laroche-Navarron - Fiche n°6 Frénantol - Les avortements spontanés à répétition, Avril 1953.
- Diethylstilbestrol – New perspectives by David A. Edelman PhD. Medical Research Consultants; Inc.Chapel Hill, North Carolina, USA. ISBN 0-85200-974-7
- Afssaps - Complications liées à l’exposition in utero au diéthylstilbestrol (DES) (Distilbène®, Stilboestrol-Borne®), Actualisation 2011 [archive].
- Obstétrique – DCEM & Sages Femmes, de P. Lopes. Date de parution, 01/01/1991. ISBN, 2853851303 (réédité en 2001).
- Fabrice Rivollier. Exposition prénatale aux perturbateurs endocriniens et risque sur le neurodéveloppement : étude de fratries exposées au diéthylstilbestrol. Médecine humaine et pathologie. 2015. ffdumas01213114f
- Perrine Planchon. Les perturbateurs endocriniens dans les produits de santé. Sciences pharmaceutiques. 2014. ffdumas-01081489f
- L'Obs, par Nathalie Bensahel. Marie-Pierre : "J'ai longtemps été blâmée, humiliée". 2015.
- L'affaire Distilbène ou Mutilations sur ordonnances, Dane Morin-Delacroix. Éditions Edilivre.
- [Online] DES Centrum – Groeiremming bij lange meisjes – Leander Wemmenhove – Najaar 2000
- Nora Cesbron - Thèse : Les SARMs (Selective Androgen Receptor Modulators) : une nouvelle famille d'agents anabolisants. Etude des effets zootechniques associés et développement de stratégies analytiques visant la mise en évidence de leur utilisation chez le jeune bovin. 07 juin 2017.
- Wuhan Demeikai Biotechnology Co., Ltd; Hongkong XinRunde Chemical Co., Ltd.
- Dr. Barbara Forney, veterinary practitioner in Chester County, Pennsylvania. Diethylstilbestrol (DES) for dogs; Stilbestrol Tablets 0.5mg drugs.com; PetCoach, Diethylstilbestrol
- Haute Autorité de Santé. DISTILBENE (diéthylstilbestrol), hormones AVIS SUR LES MÉDICAMENTS. Mis en ligne le 13 mai 2016.
- Newbold RR1, Padilla-Banks E, Jefferson WN Environmental estrogens and obesity.
- Darbre, P.D. Curr Obes Rep (2017) 6: 18. Endocrine Disruptors and Obesity